
Environmental Monitoring of Nitrates and Other Water Quality Parameters: pH,
Environmental Monitoring of Nitrates and Other Water Quality Parameters: pH,...

Chemical oxygen demand (COD) is a critical waste treatment measurement in everything from municipal systems to food manufacturing waste streams.
Performing COD testing the right way is important in determining wastewater treatment effectiveness and can help diagnose any problems in treatment. In this blog, we’ll cover what chemical oxygen demand is, how to test it, and how to get the best equipment for your tests.
Chemical oxygen demand (COD) is an indirect measurement of the amount of organic matter in a sample. With this test, you can measure virtually all organic compounds that can be digested by a digestion reagent.
COD contrasts with biochemical oxygen demand (BOD), which relies on the use of microorganisms to break down the organic material in the sample by aerobic respiration over the course of a set incubation period (typically five days).
BOD and COD correlate with one another in virtually all samples, but BOD is always lower than COD as the biochemical breakdown of organics is often not as complete as the chemical method.
As gauges of organic matter in a sample, BOD and COD are critical in wastewater for determining the amount of waste in the water. Waste that’s high in organic matter requires treatment to reduce the amount of organic waste before discharging into receiving waters.
If water treatment facilities do not reduce organic content of wastewater before it reaches natural waters, microbes in the receiving water will consume the organic matter.
As a result, these microbes will also consume the oxygen in the receiving water as part of the breakdown of organic waste. This oxygen depletion along with nutrient rich conditions is called eutrophication, a condition of natural water that can lead to the death of animal life.
Wastewater facilities reduce COD and BOD by using these same microbes under controlled conditions. These facilities aerate chambers injected with specialized bacteria that can break down the organic matter in an environment that does not harm natural waters. A reduction in BOD is used in these facilities as a benchmark for treatment effectiveness.
Since a BOD test takes five days to complete, COD is used to monitor the treatment process in day-to-day operations. The COD test takes only a few hours to complete.
If BOD were always used, treated wastewater would need to be held, and a problem with the treatment process wouldn’t be detected until five days later! This would mean that wastewater would need to be held until results could be verified.
Due to the speed of testing, facilities usually establish a correlation between bod and cod, then only run bod occasionally. however, be sure to check with your local regulatory agency for detailed advice on bod and cod testing regimens.
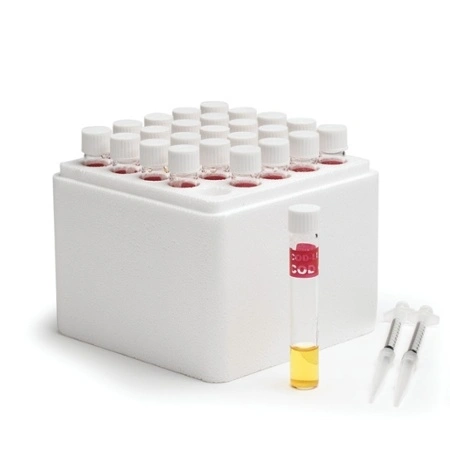
As mentioned before, COD measures organic matter by using a chemical oxidant. It’s critical that a strong enough oxidant is used to react with virtually all organic material in the sample. Historically, potassium permanganate filled this role, but it was found to be inconsistent in its ability to oxidize all the organic matter in a wide variety of waste samples. Currently, most COD tests use potassium dichromate as the oxidant. Potassium dichromate is a hexavalent chromium salt that is bright orange in colour and is a very strong oxidant. Between 95-100% of organic material can be oxidized by dichromate. Once dichromate oxidizes a substance it’s converted to a trivalent form of chromium, which is a dull green colour. Digestion is performed on the samples with a set amount of the oxidant, sulfuric acid, and heat (150°C). Metal salts are usually included to suppress any interferences and to catalyze the digestion. The digestion typically takes two hours to perform. During the digestion, it’s necessary to have excess oxidant; this ensures complete oxidation of the sample. As a result, it’s important to determine the quantity of excess oxidant. The two most common methods for this are titration and colourimetry.
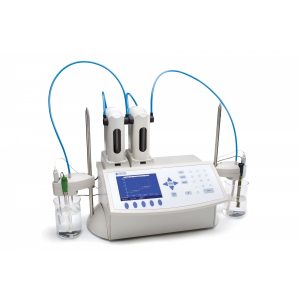
In the titration method for determining COD, the excess dichromate is reacted with a reducing agent, ferrous ammonium sulphate. As the ferrous ammonium sulphate (FAS) is added slowly, the excess dichromate is converted into its trivalent form. hi902-02_600x600As soon as all the excess dichromate reacts, an equivalence point is reached. This point means that the amount of ferrous ammonium sulphate you added is equal to the amount of excess dichromate. Color indicators can also signal this endpoint, but the process may be automated with a potentiometric indicator (like an electrode). Afterwards, you can calculate how much dichromate went towards oxidizing organic material based on how much we initially added and how much was left over.

You can also look at the consumption of dichromate by looking at the change in the absorbance of the sample. The samples absorb at particular wavelengths due to the colour of trivalent chromium (Cr3+) and hexavalent chromium (Cr6+). HI83300You can quantify the amount of trivalent chromium in a sample after digestion by measuring the absorbance of the sample at a wavelength of 600 nm in a photometer or spectrophotometer. Alternatively, the absorbance of hexavalent chromium at 420 nm can be used to determine the amount of excess chromium at the end of digestion to determine COD values.
This method is easy and requires just a few simple steps.
Both methods have their advantages and disadvantages.
Titration is less equipment-intensive since the only equipment you need is a burette, heating block, and digestion vials. However, the procedure is a little more labor-intensive. An automatic titrator can reduce the amount of user input required and can be used for other applications in wastewater (e.g. alkalinity, volatile acidity).
Although colourimetry requires a spectrophotometer or photometer, it offers convenience since most manufacturers offer premixed reagents, so all you need to do is run your samples with the digestion chemicals and minimal contact.
Colorimetry also makes measurement easy since all the analyst needs to do is digest the samples and let the instrument do the work. For these reasons, colorimetry is the most common method to measure COD.
Getting started with chemical oxygen demand requires just a few pieces of equipment. As the most common method, we’ll focus on the colourimetric method for COD. Here’s the basics of what you need:

Both methods for COD testing require the digestion step, so a heating block for your samples is crucial for ensuring accurate and repeatable results. For best results, look for a heating block that features multiple temperatures so you have utility for other tests, such as total phosphorus. Most heating blocks also have timers, which are critical for keeping digestion times consistent over multiple runs. For added safety, look for models that have an optional shield that covers the heating block in case of an accident.
The colorimeter or spectrophotometer is the device that is going to read the absorbance of the samples after digestion in order to correlate it to the COD concentration. Both of these instruments can be used to measure COD, but the two devices are a little different from one another. Colorimeters use filters to measure light as specific wavelengths, but spectrophotometers use a device that allows measurement across a wide spectrum. Regardless of which instrument you choose, look for models that feature preprogrammed methods for COD for ease-of-use.
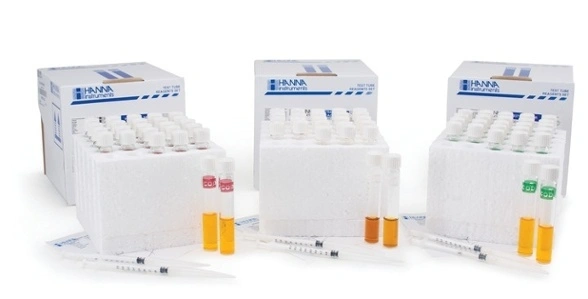
Reagents are one of the most important components of the COD testing system. These chemicals are responsible for oxidizing the organic material. It’s possible to prepare reagents in-house, but it is easier to purchase reagents to minimize contact with hexavalent chromium and strong acids. These COD vials are premixed and ready to use. There are several types of reagents available commercially:
Upgrading your COD analysis is easier than ever. Modern colourimeters and spectrophotometers have built-in methods that make the transition to a new instrument easier than ever.
Environmental Monitoring of Nitrates and Other Water Quality Parameters: pH,...
Salt Concentration In A Brine Solution For Curing Salmon Traditionally,...

To empower customers to achieve quality by supplying intuitive, accurate, and reliable analytical instruments with exceptional customer service and value.
We take pride in every product we build. From an original idea to a completed product ready for testing. We oversee every aspect of the manufacturing process. It is this level of attention to detail that sets us apart.
To empower customers to achieve quality by supplying intuitive, accurate, and reliable analytical instruments with exceptional customer service and value.
We take pride in every product we build. From an original idea, to a completed product ready for testing. We oversee every aspect of the manufacturing process. It is this level of attention to detail that sets us apart.
To empower customers to achieve quality by supplying intuitive, accurate, and reliable analytical instruments with exceptional customer service and value.
We take pride in every product we build. From an original idea, to a completed product ready for testing. We oversee every aspect of the manufacturing process. It is this level of attention to detail that sets us apart.

