
Environmental Monitoring of Nitrates and Other Water Quality Parameters: pH,
Environmental Monitoring of Nitrates and Other Water Quality Parameters: pH,...

Ever wonder what else you can do to help your plants thrive? Try testing the pH of your soil! Robust plants start from the ground up, and maintaining healthy soil is the first step towards a successful crop.
This all-encompassing guide will help anyone who is just starting out with soil pH testing. (And experienced growers will be able to learn something, too!) In the first part, we cover everything from “What is pH?” to elements and events that affect your soil’s pH, and how to fix it. After that, we go over all the different soil testing methods and tools, so you will have all the information you need to choose the best soil testing plan for you and your crops.
First, let’s go over the basics.
Water, air, and soil are the largest groups of natural resources that humans use. Soil is the loose material on the surface of the Earth that allows plants to grow. Think of the Earth as an onion with layers. Soil is only the very thin (about 200cm) top layer of the onion. All plants are grown in that thin layer.
No matter where you are on earth, soil has three main components: inorganic, organic, and microorganisms.
Five main factors affect how soil is formed: climate, organisms, an area’s geology, topography, and finally, time. With all these variables, it’s no surprise that soil varies greatly not only from state to state but even within relatively small areas.
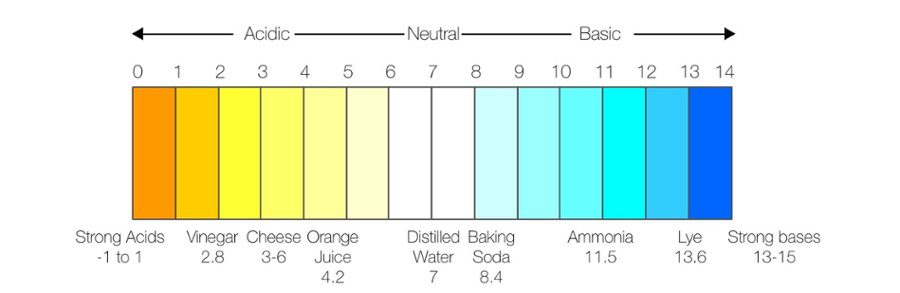
pH is a measurement of how acidic or how basic (alkaline) a substance is. When you test pH, you are measuring the number of hydrogen atoms that carry a positive charge.
The higher the concentration of hydrogen ions, the more acidic your sample is. The lower the concentration of hydrogen ions, the more basic your sample is. Acidic substances fall between pH 0 and pH 7 on the pH scale. Basic substances fall between pH 7 and pH 14 on the pH scale. pH 7 is neutral; it is neither acidic nor basic.
Common acidic substances include orange juice, soft drinks, and black coffee. Common basic items include gin, baking soda, and household cleaners. Pure water is completely neutral at pH 7.
Correct soil pH is essential to ensure optimal plant growth and crop yield because it allows nutrients to be freely available for plants to take in. Testing the pH of your soil helps to determine what plants are best suited for that area.
Sometimes soil needs supplements, like fertilisers and soil pH adjusters, for plants to be able to thrive. Measuring the pH can help you figure out what and how much you need.
Many things can affect the pH of your soil. The most common factors are climate and weather, other plants in the area, the pH of your irrigation water, soil type, the kind of fertiliser you use, and nutrient availability.
Temperature, precipitation, sunlight, and seasonal weather changes all influence soil pH. High precipitation, for example, will wash essential nutrients out of the soil. Many nutrients, such as calcium carbonates, are basic so as these nutrients leave the soil, it becomes more acidic.
Water coming into contact with decaying material in the soil (like leaves, for example) can also cause the pH to drop because decaying matter releases carbon dioxide. When carbon dioxide mixes with water, acids can form.
Drier climates or regions going through a drought will have a more alkaline soil pH. Because there isn’t as much water moving through the soil, minerals, and salts become concentrated, increasing the pH.
Native plants and local ecology can determine the starting pH of your soil. The soil underneath grasses is usually less acidic, while soils formed under trees tend to be more acidic. This is due to there being more decaying matter (leaves) near trees. The very crop you are growing can even alter your soil’s pH.
The water that you use to irrigate your crops will also influence the pH of your soil. If the water used is more acidic or more basic than the soil it is irrigating, the pH of the soil will shift.
Did the soil in your region form from granite, limestone, or shale? These parent materials will determine if your soil is more acidic or more basic. Areas with a lot of shale tend to be more acidic, while areas rich in limestone are more basic.
The texture of your soil will also determine how easy or how difficult it is to adjust the pH; this is known as the soil’s buffering capacity. Sandy soils have a lower buffering capacity while soils with more clay will have a higher buffering capacity. It’s harder to change the pH of soils with higher buffering capacities.
Fertilising soil is very important to get the best crop yields. Because pH will affect how easily available nutrients are to plants, it’s important to check soil pH before and after adding any type of fertiliser. By knowing your pH you can decide how much and what type of fertiliser you need.
Artificial nitrogen fertilisers tend to lower pH the most in soils. Organic fertiliser will acidify the soil once they come in contact with water, because of the soluble organic acids they contain.
Plants cannot absorb nutrients if the soil pH is too low or too high. When soil pH is off, nutrients such as calcium and phosphorus will bind up with other things in the soil. When the nutrients become bound up, plants will not be able to take in what they need to grow.
Most nutrients are available when the soil is slightly acidic, but different plants thrive in different pH ranges depending on their specific nutrient requirements. If the pH is too low, aluminum toxicity can occur. When this happens, aluminum becomes unbound and the plants take it in at toxic levels.
If the pH is too high, nutrients like iron become bound. Without adequate iron uptake, plants will lose their chlorophyll and start to turn yellow, indicating the plants can no longer make food for itself. Molybdenum poisoning can also occur in soils with alkaline pH, resulting in stunted crops.
Plants that thrive in more acidic soil include apple trees (pH 5 – pH 6.5), potatoes (pH 4.5 – pH 6), and orchids (pH 4.5 – pH 5.5). Alkaline-loving plants include acacia and walnut trees (they both like soil between pH 6 – pH 8).
To figure out the best pH for your needs, do a little bit of research on the type of plants that you want to grow. Natural soil is typically between pH 4 and pH 8. If your soil’s pH doesn’t match the plants’ optimal range, you’ll need to treat your soil.
Soil too acidic? Popular options for treatment are lime, calcium carbonate, and ground-up eggshells. If the soil is too basic then gypsum, iron sulphate, sulphuric acid, or calcium chloride can be added.
Irrigating the soil frequently can help lower the pH if it is too high as well. However, be careful not to over-water the plants if the treatment of the soil is in a planted area. This can cause diseases to set in, and nutrients can get diluted or washed away.
The cost of the materials and the size of the planting space will also be a factor in how you treat your soil. for example, it’s much more workable and affordable to treat a small home garden with ground eggshells than to do so in several acres of field.
The two primary ways to test soil pH from field samples are slurry testing and direct soil testing. It is important that the soil samples and tests take place in the same spots and the same way every time.
The slurry method allows you to get a representative sample and measurement of an entire area with just one test. Because soil pH can vary within a small area, be sure to take a representative sample. The soil should be taken from the same depth below the surface each time you test.
When using the slurry method, take soil from next to the plants, as well as some from further away. (Keep these two samples separate.) While this means a little extra work, you will get measurements that are more accurate since the amount of nutrients, types of soil, and moisture content can vary across a planted area.
All these things affect the pH of soil, so it’s important to track your pH at many points.
Direct soil pH testing gives you the benefit of not needing to take soil samples because the pH is tested right in the ground.
Simple!
Now that we have gone over soil and how important pH is, let’s talk about what you can use to test your soil pH. We’ve narrowed it down to four main groups: test strips, chemical soil test kits, digital pocket testers, and portable soil testing meters.
Pros: Easy to use, inexpensive
Cons: Hard to read, loss of precision, hidden costs
pH test strips (aka litmus paper) are paper strips that have been saturated with pH-sensitive dyes. When exposed to a damp substance, the strips will change colour relative to that substance’s pH. This colour change corresponds to a colour chart provided with the test strips. This method for testing is quick, easy, and inexpensive, but it does have some disadvantages.
Soil is very dark in colour, even when mixed with water. The muddy colour could stain the test strips and make them hard to read. Even when a colour change can be seen, it’s subjective since colours can look different depending on the lighting, as well as from person to person. This leads to inconsistent and poor results.
Test strips will not give the most accurate test results either; they only have a resolution of 0.5 pH units. This means the closest your test results can get to your soil’s true pH would be 0.5 pH +/-. Being 0.5 pH units off means a greater cost to treat the soil. If the soil treatment is not accurate you can have low crop yield and dead plants.
Pros: Easy to use, all-inclusive
Cons: Multiple kits needed, hard to read & dispose of, a limited number of tests
pH chemical test kits are like test strips in that they are easy to use, but also have several drawbacks. Using a soil test kit involves adding your soil, distilled or DI water, and some chemicals (these will be included when you purchase a kit) to a tube. The chemicals, like test strips, react with the pH levels in your sample to create a colour change. Also like test strips, the colour change of the test kits will be subjective, and readings will vary between different people.
pH test kits have lower resolution, generally between 1 or 0.5 pH points, and tend to test specific ranges of pH. That means you need to buy many kits to test your different types of soil, or when you are just beginning and don’t know your starting pH.
The number of tests you perform with a chemical test kit limited to the number of reagents included. Regular kits include enough chemicals for anywhere between 1 and 25 tests. Disposal of these chemicals poses another issue; chemical compositions differ from manufacturer to manufacturer, and many cannot just be poured down the drain or into the trash.
Pros: Pocket size, better accuracy, easy to keep clean
Cons: Need to know how to care for the device
Soil pH pocket testers are digital, portable testing instruments that utilize a pH electrode. The integration of a pH electrode in the durable casing of a tester allows for much greater accuracy than test kits or strips. The pH electrode takes a pH reading in your soil or soil slurry and displays it on an LCD screen.
Testers have fewer things to interfere with taking a reading compared to test kits and strips. You no longer have to worry about the dark soil sample interfering with colour change, or the subjectivity of color change tests in general. Many testers also have a much higher resolution and accuracy than chemical options, generally between 0.1 and 0.01 pH units.
Soil pH testers allow for easy testing out in the field, with simple, single-handed, one- or two-button functions. Some testers have special features like waterproof capabilities or durable bodies which let you test in humid environments without affecting the readings.
Certain pocket testers also feature a cloth junction, which helps prevent clogging of the electrode. To unclog soil particles from the junction, the cloth can be gently tugged with tweezers to remove debris and uncover fresh cloth in the junction. This gives you more accurate readings and longer electrode life.
As the temperature changes, the way the electrode behaves will also change; this can affect your pH readings. To help counter this, some pH soil testers have automatic temperature compensation, a feature that allows the device to correct the error.
These handy instruments do take a little bit more know-how to operate and properly care for. You’ll need solutions to keep the electrode calibrated, hydrated, and cleaned. More information on care for a pH electrode can be found under Care and Maintenance below.
Pros: Portable laboratory accuracy, customizable, no more guesswork
Cons: Larger investment, more technical
Portable soil pH meters are the next step up from soil pH testers. They are a convenient way to have laboratory accuracy in field testing. A bit larger than the testers, portable soil pH meters offer many functions from multiparameter testing to data logging.
All choice portable soil pH meters have automatic temperature compensation; they will come with either an integrated temperature sensor, or a separate temperature probe. Portable soil pH meter measurements are nearly exact, with resolutions as low as 0.001 pH units. Both of these functions give you readings with much greater accuracy.
If you need to report your pH values, a portable soil pH meter is a great choice. Some of these meters are able to provide Good Laboratory Practices (GLP) data, which includes things such as date, time, calibration data, and logged data. This gives traceability to your readings.
Certain soil pH meters come with an electrode diagnostics feature called CAL Check™. The meter will check the condition of the electrode and pH buffers during calibration. Some of these meters also come with a HELP button which will bring up tutorials right on the screen.
Looking for something smaller than a typical portable soil pH meter, but need the accuracy of one? Some portable soil pH meters do not even need a regular meter! There are electrodes on the market that can wirelessly link to a smartphone or tablet.
Proper care and maintenance of your pH electrode is essential. Appropriate care of electrodes will extend its useful life. Our maintenance motto will help you to remember the three main concepts in electrode maintenance: Clean Regularly, Calibrate Often, and Condition Always.
When testing the pH of soil, it’s important to properly clean the pH electrode since soil can clog the junction. If soil gets stuck on the electrode, do not wipe it! Instead, rinse the electrode with distilled water.
Is the soil really sticking to the electrode? Soak it in a cleaning solution specially formulated for soil or humus deposits . Both solutions help to remove residue left behind after rinsing the electrodes with distilled water. The cleaning solution for soil deposits is great for general agricultural samples; the humus solution is the best for highly organic soils (such as compost).
Cleaning the electrode will give you maximum efficiency and accuracy when taking pH readings. After using a cleaning solution, the electrode should be placed in a storage solution for at least one hour before using it again. The cleaning solutions are available in disposable one-use-only packets as well as bottles.
Calibrating your electrode will give you the greatest accuracy when testing pH. Calibration will help to correct your electrode as its response changes over time, due to aging and other factors.
Electrode response changes due to several factors. It’s important to calibrate to at least two pH points which brackets your expected pH value. Bracketing simply means calibrating to one pH point below the expected range, and one pH point above the expected range. (For example, if your expected reading is pH 8.6, then pH 7 and pH 10 buffers should be used.)
The most important part of the pH electrode is the sensing bulb at the bottom. The bulb is made of glass that is sensitive (responsive) to hydrogen ions. It’s important to maintain equilibrium in the electrode to keep your readings stable by keeping the bulb hydrated.
Proper hydrating means always storing your electrode in a storage solution. Storage in other liquids, like distilled or DI water, can damage the glass bulb and cause slow, inaccurate pH readings.
Environmental Monitoring of Nitrates and Other Water Quality Parameters: pH,...
Salt Concentration In A Brine Solution For Curing Salmon Traditionally,...

To empower customers to achieve quality by supplying intuitive, accurate, and reliable analytical instruments with exceptional customer service and value.
We take pride in every product we build. From an original idea to a completed product ready for testing. We oversee every aspect of the manufacturing process. It is this level of attention to detail that sets us apart.
To empower customers to achieve quality by supplying intuitive, accurate, and reliable analytical instruments with exceptional customer service and value.
We take pride in every product we build. From an original idea, to a completed product ready for testing. We oversee every aspect of the manufacturing process. It is this level of attention to detail that sets us apart.
To empower customers to achieve quality by supplying intuitive, accurate, and reliable analytical instruments with exceptional customer service and value.
We take pride in every product we build. From an original idea, to a completed product ready for testing. We oversee every aspect of the manufacturing process. It is this level of attention to detail that sets us apart.

