Bromine
In many countries bromine sanitising has been introduced as an alternative to chlorine, although it is not as strong. The advantage of bromine lies in its stability at higher temperatures (advantageous for heated pools and hot tubs), and its maintained disinfection power at a higher pH. Furthermore, there is a very little reaction between bromine and nitrogen compounds, reducing the unpleasant odour, and eye irritation problems. The main disadvantage of bromine is the slower acting disinfecting power, making it less suitable for larger pools.
Ozone
Ozone is a very strong oxidising agent that destroys organic compounds that are especially difficult to oxidise. It allows the pool manager to very efficiently remove combined chlorine without frequently refreshing large amounts of pool water. By the time the water passes through the filter units, ozone has already completed sanitising, and it is not affected by the pH level.
Mainly because of its strong oxidising power, the return water may contain trace concentrations of ozone. It imperative to know that ozone is very unstable, so to ensure thorough sanitization of the water, low-level chlorination remains necessary.
Calcium
The presence of calcium in the system is desired to ensure filming on those places where the temperature is relatively high, like in boilers and pipes transporting warm water. Scaling must be avoided because it reduces heat transfer and pump capacity, and causes cloudiness in the water.
It is recommended to maintain the calcium hardness value within the range from 200 to 400 ppm as calcium carbonate (CaCO₃).
Alkalinity
Alkalinity is the measure of the total concentration of alkaline substances, mostly bicarbonates, dissolved in the water. The higher the alkalinity, the more resistant the water is to pH change. At the same time, high alkaline water is a major contributor to scaling problems like incrustation in filtration equipment, pumps, and piping.
It is recommended to maintain the alkalinity value within the range from 80 to 125 ppm as calcium carbonate (CaCO₃).
The pH of the water is an important factor since at lower pH levels the corrosion rate increases. If the alkalinity values are sufficiently high, it will not be difficult to control the pH. Most pool managers prefer to keep the pH between 7.2 and 7.4 to best maintain low corrosion rates and a sufficient activity of chlorine.
Temperature
Microorganisms grow more rapidly at higher temperatures, so the required sanitisation level for a swimming pool or spa is highly dependent on the water temperature; this is especially concerning for heated pools and spas. A general rule of thumb is that for every 5.5°C increase in water temperature over 27°C, the concentration of required sanitizer is doubled.
ORP (Redox)
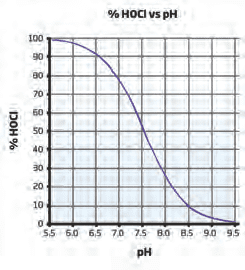
While free chlorine is a measure of the quantity of available chlorine present in pool and spa water, other water properties may affect the ability of that chlorine to properly disinfect. A sufficient concentration of free chlorine may be present in solution, but based on certain properties it may not be effectively sanitising. Oxidation-reduction potential (ORP) directly measures how strong the oxidising potential in a solution is, thus determining the sanitation effectiveness. Two pools with the same free chlorine concentration may have completely different ORP values based on pH, cyanuric acid concentration, and temperature. Therefore, ORP is the best indication of how effectively the pool or spa water is being sanitised. While optimal concentration levels vary based on the type of sanitizer being used, the ORP level should be maintained above 650 mV for pools and spas treated with chlorine, bromine, or iodine.
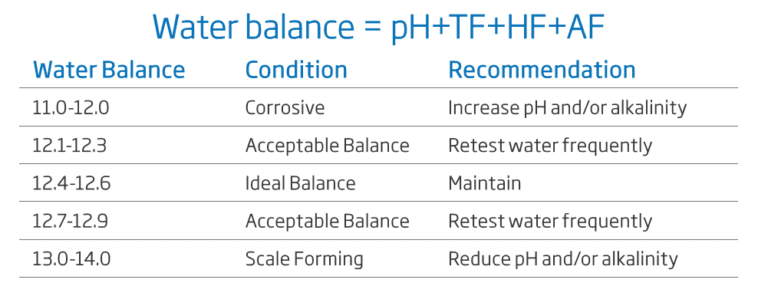
The Water Balance and Langelier Index
Pool water characteristics need to be maintained in a balanced state to avoid numerous issues. Measuring certain variables is extremely important to predict if the water is corrosive or will cause scaling.
A saturation index developed by Dr. Wilfred Langelier is widely used to predict the balance of swimming pool waters (pool test kit). It represents the estimation of a solutions ability to dissolve or precipitate calcium carbonate deposits. A certain level of this precipitation (filming) is desired to insulate pipes and boilers from contact with water. When no protective filming is formed, water is considered to be corrosive. On the other hand, too much filming can develop into scaling and incrustation of the pipes. In the treatment and monitoring of pool water, the pool manager must ensure that related parameters such as alkalinity, hardness and pH are carefully monitored in addition to sanitising chemicals.
The Langelier Index is a powerful tool to calculate the water balance, and to predict corrosion or scaling problems. Theoretically, a LI of zero indicates perfect water condition for swimming pools. If LI>0, scaling and staining of the water is present, and if LI<0 the water is corrosive and highly irritating. A tolerance of ±0.4 is normally acceptable.
The Langelier formula is expressed as: LI = pH + TF + HF + AF – 12.5
Where:
LI = Langelier Index (also called Saturation Index)
pH = pH of the water
TF = temperature factor
HF = hardness factor, log (Ca hardness, ppm as CaCO)
AF = alkalinity factor, log (alkalinity, ppm as CaCO)
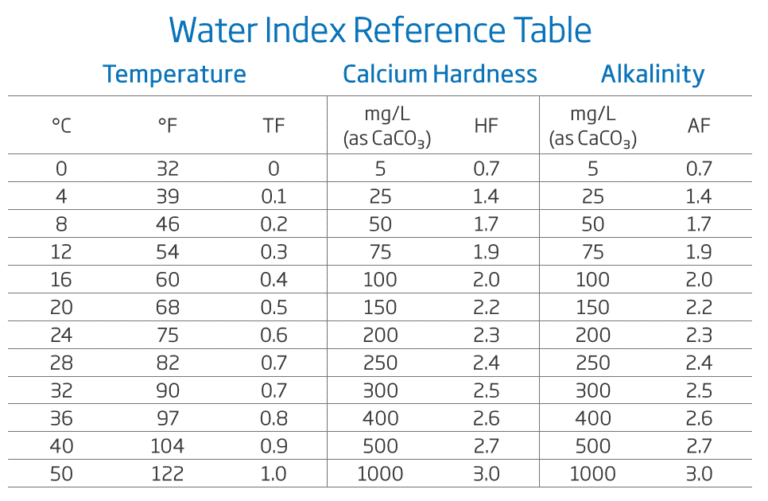
To calculate the exact Langelier Index of your water please use the WATER INDEX reference tables.
For most pools, water is balanced if:
• The pH value is maintained within the recommended ranges of pH 7. 2 – 7. 6
• Ideally, the Alkalinity should be maintained within a range of 80 – 125 ppm
• The Calcium Hardness should be maintained within a range of 200 – 400 ppm.
To calculate your water balance, three parameters must be measured; calcium hardness, alkalinity and pH. Find the hardness and alkalinity factor in the reference tables below.
The water temperature is, in general, maintained between 24°C and 34°C. Assuming the temperature is kept within those ranges, an average value or 0.7 may be used.