Hanna Instruments offers a wide variety of pH electrodes that are designed for many different applications. The type of glass used for sensing pH, bulb shape, body material, type of junction, type of reference and electrolyte used are just some of the design considerations.
The HI729113 uses general purpose (GP) glass, flat tip bulb, titanium body, and PTFE junction with polymer electrolyte.
General Purpose Glass Formulation
The measurement of pH at very high temperatures is detrimental to the sensitive glass bulb and will shorten the life of it. A pH electrode with general purpose (GP) glass will have a resistance of 100 megaohms at 25℃ while the resistance of LT glass is around 50 megaohms at 25℃. As the temperature of the glass decreases in the sample, the resistance of the LT glass will approach that of GP glass. If using GP glass, the resistance would increase above the optimum range, resulting in increased impedance and ultimately affecting the measurement. The HI729113 is suitable to use with samples that measure from 0 to 80℃.
Flat Tip Bulb
The recessed flat tip bulb of the HI729113 is easy to clean and prevents solids in solutions from collecting on the sensor. Other tip shapes include conic for penetration and spheric for aqueous measurements.
Titanium Body
A titanium body increases immunity to electrostatic and magnetic fields. It also allows for strong corrosion resistance, even in seawater.
Matching Pin
A matching pin is a differential measurement technique used to eliminate ground loops and common mode perturbations for the measurement system. In a system without a matching pin, electrical currents in the sample can affect the reference half cell voltage that is connected via the liquid junction with the sample. In this case, the reference electrode picks up the electromagnetic fields and the measurement of the pH is altered. The matching pin isolates these current/magnetic fields from the reference electrode. The HI729113 titanium body serves as a matching pin for safe precise pH measurements.
PTFE Junction
Porous polytetrafluoroethylene (PTFE) is a hydrophobic material that is available with different porosities. This type of junction is often used on electrodes with polymer electrolytes. Because of its chemical advantages, PTFE is widely used in industrial applications.
DIN Connector
The HI729113 uses a quick connect DIN connector. This type of connector is generally proprietary to the meters they are supplied with and may not be interchangeable. The HI729113 is designed for use with Hanna’s HI99141 pH meter. Other type of connectors include BNC, screw type, T-type, and 3.5mm to name a few.
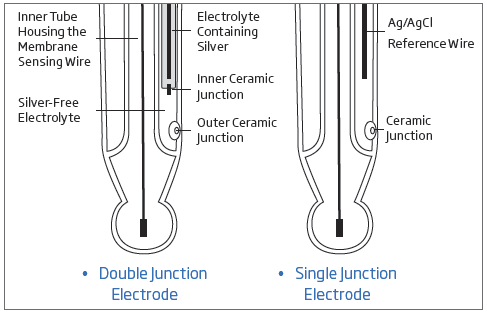
Single Junction Versus Double Junction pH Electrodes
Conventional electrodes are normally single junction. As depicted by the figure above, these electrodes have only a single junction between the internal reference wire and the external solution. Under adverse conditions, such as high pressure, high temperature, highly acidic or alkaline solutions, the positive flow of the electrolyte through the junction is often reversed resulting in the ingress of sample solution into the reference compartment. If this is left unchecked, the reference electrode can become contaminated, leading to complete electrode failure. Another potential problem with single junction electrodes is the clogging of the junction due to silver chloride (AgCl) precipitation. Silver can be easily precipitate in samples that contain Tris buffer or heavy metals. When the electrolyte solution makes contact with the sample, some AgCl will precipitate on the external face of the junction. The result is drifty readings obtained from the sensor.
Hanna’s double junction system, as the name implies, has two junctions, only one of which is in contact with the sample as shown in the figure. Under adverse conditions, the same tendency of sample ingress is evident. However, as the reference electrode system is separated physically from the intermediate electrolyte area, the contamination of the electrode is minimized. The likelihood of clogging of the junction is also reduced with a double junction electrode since the outer reference cell uses a fill solution that is “silver-free”. Since there is no silver present, there is no precipitate that can form to clog the junction.
All prices are inclusive of GST and not all items are stock items, if you require an immediate solution please send an email to sales@hannainst.com.au or call us on (03) 9769 0666.
Free standard delivery: We offer free delivery within Australia on orders over $100, typically arriving within 5 to 7 days. Delivery times may vary depending on the courier service and the recipient's location. Free shipping is not available with other offers or discounts.
Same-day despatch: Place your order before 1 PM Monday to Friday for same-day despatch. Delivery times may vary depending on the courier service and the recipient's location. Subject to stock availability.
Please note that we do not offer shipping to PO Box addresses.